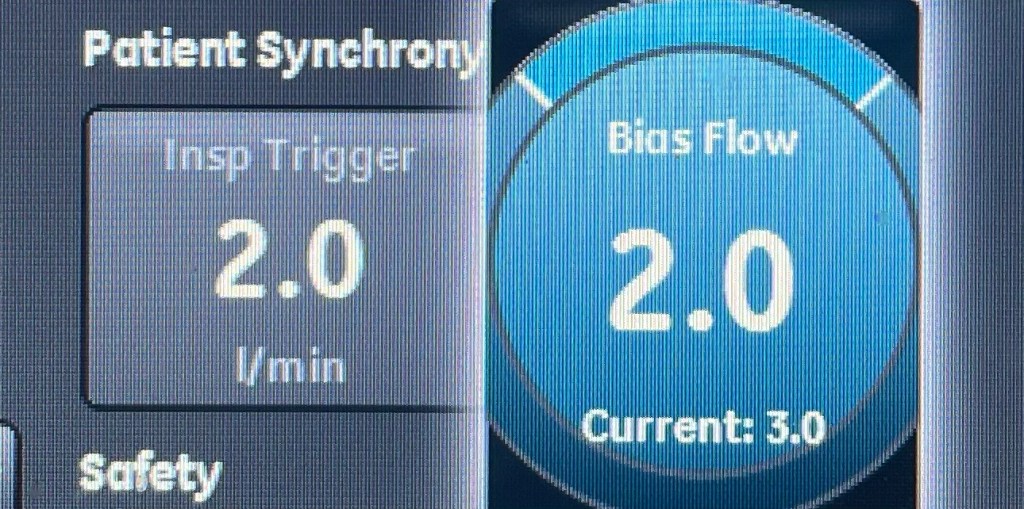
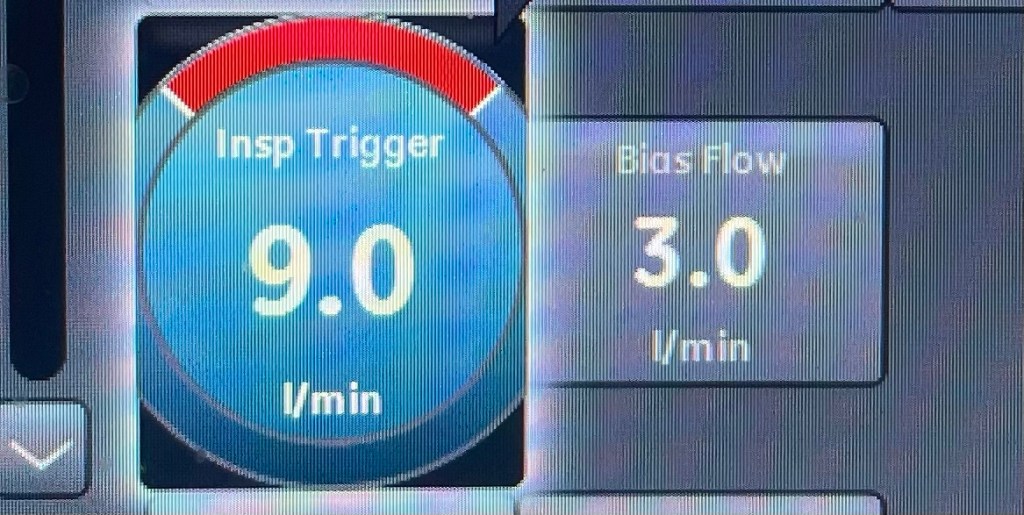
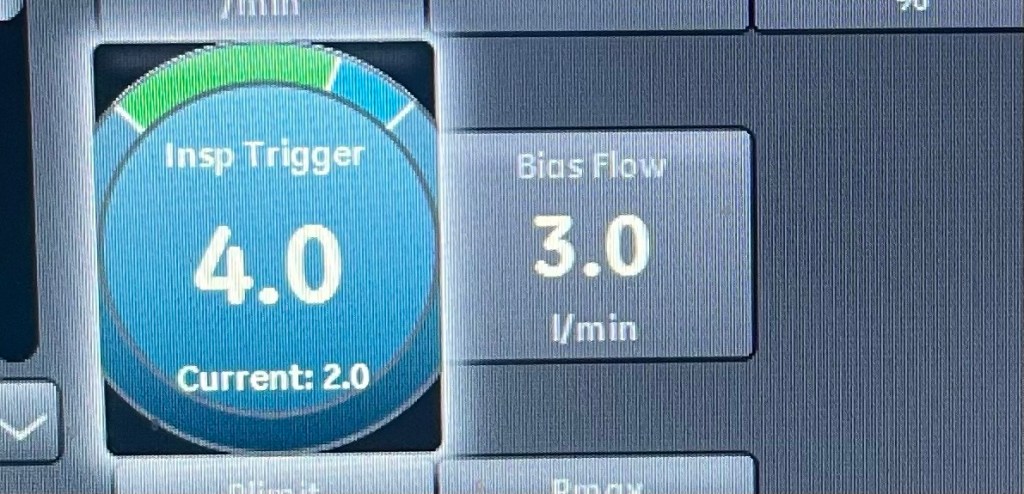
Introduction: The Complex Role of Protein in ICU Patients
For decades, ICU nutrition guidelines emphasized high protein intake (≥1.5 g/kg/day) to combat muscle wasting, malnutrition, and immune dysfunction in critically ill patients. However, recent landmark RCTs and meta-analyses have challenged the belief that “more protein is always better.”
Definition of Sarcopenia
• Consensus Definitions:
Major working groups such as the European Working Group on Sarcopenia in Older People (EWGSOP) and the Asian Working Group for Sarcopenia (AWGS) define sarcopenia as a condition involving both low muscle quantity and low muscle function (either strength or performance).
• Probable Sarcopenia: Identified by low muscle strength (commonly measured by handgrip strength).
• Confirmed Sarcopenia: When low muscle mass is also present.
• Severe Sarcopenia: When there is low muscle mass, low muscle strength, and poor physical performance (e.g., slow gait speed).
Methods to Measure Sarcopenia
1. Imaging Techniques for Muscle Mass
• Dual-Energy X-ray Absorptiometry (DXA):
• Pros: Widely used; provides detailed body composition analysis.
• Cons: Requires patient transfer and specialized equipment; less practical in critically ill patients.
• Computed Tomography (CT) and Magnetic Resonance Imaging (MRI):
• Pros: Considered gold standards for measuring muscle cross-sectional area; excellent image quality.
• Cons: Exposure to radiation (CT), high cost, and impractical for repeated bedside evaluations.
• Ultrasound:
• Pros: Non-invasive, portable, and repeatable at the bedside.
• How It’s Done:
• Site: The rectus femoris muscle of the thigh is commonly used.
• Technique:
• Patient Positioning: Patient is in a supine position with the knee extended and relaxed.
• Landmarks: Measure at the midpoint between the anterior superior iliac spine (ASIS) and the patella.
• Probe: A high-frequency linear probe (7–12 MHz) is used.
• Measurements:
• Muscle Thickness (RF-MLT): Distance between the superficial and deep fascia layers of the muscle.
• Cross-Sectional Area (RF-CSA): Outline the muscle boundary to calculate area.
• Interpretation: A reduction of more than 10% in muscle thickness over 7 days is often considered indicative of significant muscle wasting, which correlates with sarcopenia and poorer outcomes.
• Bioelectrical Impedance Analysis (BIA):
• Pros: Quick and easy; provides estimates of fat-free mass.
• Cons: Accuracy can be affected by fluid shifts common in critically ill patients.
2. Functional Measures
• Handgrip Strength:
• Method: Use a dynamometer to measure the maximum voluntary contraction of the hand.
• Thresholds (EWGSOP2): Common cutoffs are <27 kg for men and <16 kg for women in older adults, though ICU-specific thresholds might differ.
• Significance: Reduced grip strength is a strong predictor of sarcopenia and overall functional decline.
• Physical Performance Tests:
• Tests such as gait speed or the Short Physical Performance Battery (SPPB) are used in ambulatory patients but are often not feasible in critically ill, immobilized patients.
Because sarcopenia encompasses both muscle mass and muscle function, the diagnosis is typically made by combining measurements:
• Step 1: Screening for low muscle strength (e.g., low handgrip strength).
• Step 2: Confirming low muscle mass through imaging (ultrasound, DXA, CT) or body composition analysis (BIA).
• Step 3: Assessing physical performance (if possible), to gauge the severity.
Integration with Nutritional Assessments:
Tools like GLIM, NRS-2002, and mNUTRIC incorporate various parameters (e.g., weight loss, BMI, decreased food intake, severity of illness) to assess nutritional risk. Although these tools have limitations in the ICU (due to fluid shifts and rapid metabolic changes), they can be complemented by direct muscle measurements to provide a fuller picture of a patient’s nutritional and functional status.
• Implications for Treatment:
Early identification of sarcopenia through ultrasound and functional tests can lead to timely interventions, such as adjusting protein intake, initiating early mobilization, and using anabolic agents or nutritional supplements (e.g., HMB) to preserve muscle mass.
This article will cover:
✅ What does the latest research say about protein intake in ICU patients?
✅ How does malnutrition impact ICU outcomes, and how can we assess it?
✅ Why might excessive protein be harmful in conditions like AKI?
✅ What are the molecular mechanisms of muscle wasting in ICU patients?
✅ How can bedside ultrasound help monitor muscle loss?
✅ What is the optimal protein dosing strategy for different ICU populations?
By the end, we’ll establish a personalized, evidence-based approach to ICU protein nutrition.
1. The Burden of Malnutrition in ICU Patients
Malnutrition is common in ICU patients due to:
🔹 Hypermetabolism & catabolic stress (sepsis, trauma, burns)
🔹 Reduced oral/enteral intake (NPO status, GI dysfunction)
🔹 Muscle breakdown from immobilization & inflammation
📊 Key Facts:
• Up to 60% of ICU patients develop malnutrition within 48 hours of admission
• Malnourished ICU patients have:
🚨 Higher mortality (30–50%)
🚨 Longer mechanical ventilation & ICU stay
🚨 Greater risk of ICU-acquired weakness (ICUAW)
How Do We Assess Malnutrition in ICU Patients?
🔹 1. GLIM Criteria (Global Leadership Initiative on Malnutrition)
📌 Two-Step Diagnosis:
1️⃣ Phenotypic Criteria (Need at least 1)
• Weight loss (>5% in 6 months or >10% in 1 year)
• Low BMI (<20 if <70 years, <22 if >70 years)
• Reduced muscle mass (via ultrasound, CT, bioimpedance)
2️⃣ Etiologic Criteria (Need at least 1)
• Reduced food intake/absorption (>50% of needs for >1 week)
• Systemic inflammation (sepsis, burns, major surgery)
🚀 Patients meeting 1 phenotypic + 1 etiologic criterion = Malnutrition diagnosis
🔹 2. NRS-2002 (Nutritional Risk Screening – 2002)
📌 Used in ICU to screen for malnutrition risk
🔹 Scoring System (0–6 points):
✔ Nutritional Status (0-3 points) → Weight loss, BMI, intake reduction
✔ Disease Severity (0-3 points) → Sepsis, major surgery, ICU stay
✔ Age ≥70 years → Add +1 point
📌 Scores ≥3 indicate malnutrition risk → Early nutrition therapy needed
🔹 3. mNUTRIC Score (Modified Nutrition Risk in Critically Ill)
📌 Designed for ICU patients to predict mortality risk
🔹 Includes:
✔ APACHE II / SOFA score
✔ Age, BMI, number of comorbidities
✔ Days from ICU admission to nutrition initiation
🚀 mNUTRIC ≥5 = High risk → Early aggressive nutrition needed
2. ICU-Acquired Weakness (ICUAW): Causes & Prevention
What Causes ICUAW?
🚨 Critical illness leads to a combination of:
• Inflammation-induced muscle breakdown (TNF-α, IL-6, IL-1β)
• Corticosteroid-induced myopathy
• Mitochondrial dysfunction
• Insulin resistance & anabolic resistance
• Disuse atrophy from bed rest
📊 Up to 50% of ventilated ICU patients develop ICUAW, leading to:
🚨 Delayed ventilator weaning
🚨 Longer ICU stay
🚨 Increased 1-year mortality
3. Molecular Mechanisms of Muscle Loss in ICU Patients
✅ Muscle protein synthesis is impaired due to:
• mTOR inhibition by inflammatory cytokines
• Suppression of IGF-1 (growth factor needed for muscle regeneration)
🚨 Muscle breakdown is accelerated due to:
• Activation of Ubiquitin-Proteasome System (UPS)
• Mitochondrial dysfunction leading to oxidative stress
🔬 Anabolic resistance → Even with high protein intake, ICU patients fail to utilize amino acids efficiently.
4. What Do Major Clinical Trials Say About ICU Protein Needs?
Major Clinical Trials: The High-Protein Controversy
🔹 EFFORT Trial (2023, The Lancet)
“More Protein Didn’t Improve Outcomes—And May Have Harmed Some Patients.”
• Study Design: Large multicenter RCT (85 ICUs, 1,301 patients)
• Intervention: High protein (2.2 g/kg/day) vs. Standard protein (1.2 g/kg/day)
• Primary Outcome: No difference in time-to-discharge-alive (p=0.27)
🔍 Key Findings:
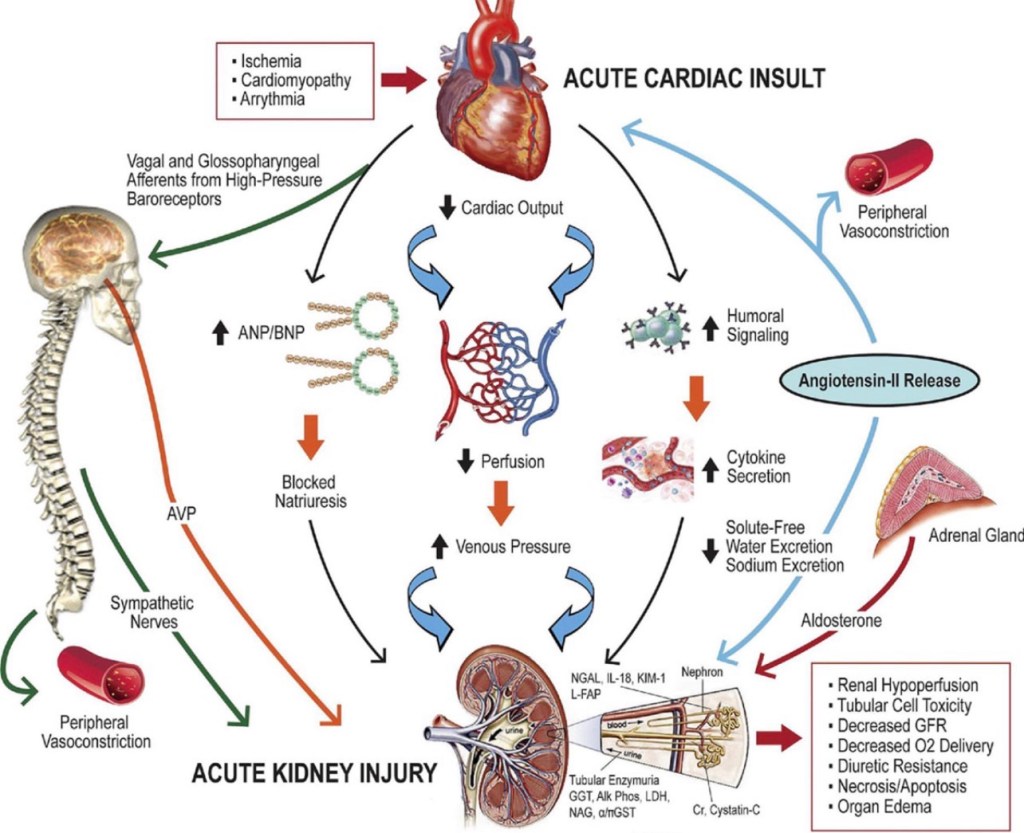
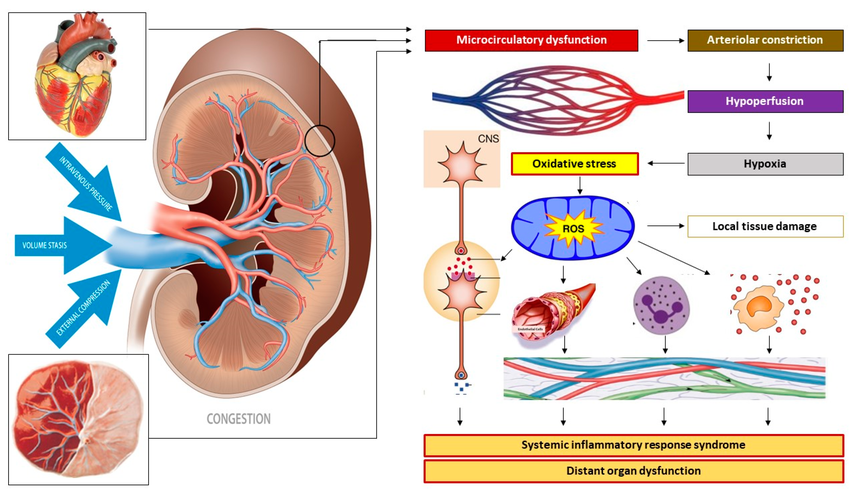
🚨 High protein worsened outcomes in AKI patients (HR 1.12, 95% CI: 1.02–1.27)
🚨 No benefit in mortality, ventilator-free days, or infections
📌 Takeaway: Very high protein (>2.2 g/kg/day) might be harmful, especially in AKI & multi-organ failure patients.
🔹 TARGET Trial (2018, NEJM)
“More Calories Did Not Improve Survival.”
• Study Design: Multicenter RCT (3,957 ICU patients)
• Intervention: High-energy EN (1.5 kcal/ml) vs. Standard EN (1 kcal/ml)
• Primary Outcome: No difference in 90-day mortality
🔍 Key Findings:
❌ Higher calorie intake did not reduce ICU mortality or ventilator duration
❌ No reduction in infections or complications
📌 Takeaway: Caloric overfeeding is ineffective—protein needs should be separately optimized.
🔹 NUTRIREA-2 Trial (2018, JAMA)
“Route of Feeding Matters More Than the Protein Dose.”
• Study Design: Multicenter RCT (2,400 ICU patients)
• Intervention: Early Enteral Nutrition (EN) vs. Early Parenteral Nutrition (PN)
• Primary Outcome: No difference in 28-day mortality
🔍 Key Findings:
🚨 EN group had more GI complications (vomiting, diarrhea, aspiration pneumonia)
🚨 PN group had more bloodstream infections
📌 Takeaway: EN remains the preferred route, but careful monitoring for GI intolerance is needed.
🔹 2024 Meta-Analysis (Lee et al.)
“High Protein Increased Mortality in AKI Patients.”
• Data: 23 RCTs, 3,303 ICU patients
• Primary Outcome: No mortality benefit of high protein overall
🔍 Key Findings:
🚨 Increased mortality in AKI patients (42% higher risk, NNH = 7)
✅ High protein preserved muscle mass but increased nitrogen waste
📌 Takeaway: Moderate protein (~1.2–1.5 g/kg/day) is safest, while AKI patients should receive ≤1.2 g/kg/day.
However, excessive protein intake may:
❌ Increase metabolic waste (urea, nitrogen load), worsening AKI
❌ Cause unintended hyperglycemia (via gluconeogenesis)
❌ Fail to translate into better survival outcomes (as seen in recent trials)
Study
Protein Intake
Key Findings
EFFORT (2023, Lancet)
2.2 g/kg/day vs. 1.2 g/kg/day
Higher protein increased mortality in AKI patients (p=0.005) 🚨 No benefit in ICU/hospital stay ❌
TARGET (2018, NEJM)
High-calorie EN (1.5 kcal/ml) vs. Standard EN (1 kcal/ml)
No survival benefit of increased calorie intake ❌
NUTRIREA-2 (2018, JAMA)
Early EN vs. Early PN
No difference in mortality, but EN caused more GI issues ❌
Meta-Analysis (2024, Lee et al.)
1.5 g/kg/day vs. 0.92 g/kg/day
🚨 High protein increased AKI mortality by 42% (NNH = 7) ❌ No mortality benefit overall
📌 Takeaway: Moderate protein (1.2–1.5 g/kg/day) is safest, while AKI patients should receive ≤1.2 g/kg/day.
5. Bedside Ultrasound for Muscle Monitoring in ICU
✅ Rectus Femoris ultrasound (RF-US) is the best tool for real-time muscle loss assessment.
📊 How to interpret:
📉 >10% muscle loss in 7 days → ICUAW risk
📉 >15% loss over ICU stay → Long-term disability risk
🔬 Combining nutrition + rehab (electrical stimulation, resistance exercise) improves muscle preservation.
6. Practical ICU Protein Guidelines Based on Current Evidence
Patient Type
Protein Intake
General ICU Patients
1.2–1.5 g/kg/day
Sepsis, Trauma, Burns
1.5–2.5 g/kg/day
AKI (No CRRT)
≤1.2 g/kg/day
CRRT (Dialysis Patients)
2.0–2.5 g/kg/day
The ESPEN guidelines on clinical nutrition in the ICU provide several key recommendations on protein intake, timing, and considerations for different patient groups. Here are the most relevant points extracted from the document:
Protein Intake Recommendations
• General ICU Patients: ESPEN recommends 1.3 g/kg protein equivalents per day during critical illness, delivered progressively .
• Sepsis & Septic Shock: ESPEN does not provide a single definitive target but suggests avoiding overfeeding, as septic patients may not benefit from increased protein intake (1.2 g/kg/d) compared to non-septic patients, though no harm was reported either .
• Acute Kidney Injury (AKI): Traditional recommendations suggest 1.2-1.5 g/kg/d, but some studies indicate that higher protein intake might be associated with worse outcomes in AKI patients not receiving renal replacement therapy .
• Sarcopenic ICU Patients: Those with muscle loss may benefit more from >1.2 g/kg/day protein intake .
• Parenteral Nutrition (PN): ESPEN suggests that PN should be used if enteral nutrition (EN) is insufficient after three days. Overfeeding should be avoided in the first days of ICU admission
1. Acute Phase of Sepsis:
During the initial acute phase (first 24–96 hours), it’s recommended to provide a protein intake of approximately 1.0 g/kg/day. This approach aims to balance the need for protein while avoiding potential adverse effects associated with higher protein delivery during this critical period.
2. Post-Acute Phase of Sepsis:
After the acute phase, as the patient stabilizes, increasing protein intake becomes crucial to minimize muscle loss and support recovery. Guidelines suggest aiming for a protein intake of 1.2–2.0 g/kg/day during this period.
3. Recovery Phase:
In the recovery phase, particularly for patients engaging in rehabilitation and physical therapy, protein requirements may further increase. Some recommendations advocate for protein intakes up to 2.0 g/kg/day to support muscle rebuilding and overall recovery.
Clinical Evidence Supporting Higher Protein Intake:
• A retrospective study observed that patients with sepsis who received higher protein intake during the first week had a lower in-hospital mortality rate. Specifically, an average daily protein intake was associated with a reduced risk of in-hospital death.
• The ESPEN guidelines on clinical nutrition in the ICU highlight that protein intakes greater than 1.2 g/kg/day are associated with improved outcomes in critically ill patients, including those with sepsis.
It’s important to note that while higher protein intake is beneficial during the post-acute and recovery phases, individual patient factors such as kidney function, metabolic status, and overall clinical condition should guide specific protein targets. Close monitoring and adjustment of nutritional strategies are essential to optimize outcomes in septic patients.
🚀 The future isn’t “more protein” but “right protein, right patient, right time.”
📌 Final Thought: ICU nutrition must be tailored—not just based on general guidelines, but on real-time patient responses.
Extras
Detailed Overview of HMB (β-Hydroxy β-Methylbutyrate) in ICU Patients
HMB (β-Hydroxy β-Methylbutyrate) is a metabolite of leucine that has been widely studied for its muscle-preserving and anabolic effects in critically ill patients. Below is a comprehensive review of its mechanism of action, clinical benefits, side effects, and landmark studies supporting its use.
1. Mechanism of Action: How HMB Works in ICU Patients
HMB exerts multiple beneficial effects in critically ill, immobilized, and muscle-wasting patients through the following mechanisms:
(A) Stimulation of Muscle Protein Synthesis (mTOR Activation)
✅ HMB activates the mTOR pathway, which is the key regulator of muscle growth.
✅ This leads to increased muscle protein synthesis (MPS) → Helps maintain lean body mass.
✅ Unlike leucine, HMB is more potent in stimulating MPS because it bypasses certain metabolic steps.
📌 Supporting Study:
• Eley et al. (2008): HMB increased muscle protein synthesis by 30% in septic rats.
(B) Inhibition of Muscle Protein Breakdown (Ubiquitin-Proteasome Pathway Suppression)
✅ Critical illness activates the Ubiquitin-Proteasome System (UPS) → leading to muscle proteolysis (breakdown).
✅ HMB directly inhibits UPS, preventing excessive protein degradation.
✅ This helps preserve muscle mass even during immobilization or sepsis.
📌 Supporting Study:
• Smith et al. (2005): HMB reduced muscle protein breakdown by 50% in cachectic cancer patients.
(C) Anti-Inflammatory Effects (↓ TNF-α, IL-6, IL-1β)
✅ Critical illness triggers systemic inflammation (sepsis, trauma, burns), leading to muscle wasting.
✅ HMB reduces pro-inflammatory cytokines (IL-6, TNF-α, IL-1β) → Improves muscle retention.
✅ HMB also preserves mitochondrial function, reducing oxidative stress.
📌 Supporting Study:
• Hsieh et al. (2006): ICU patients receiving HMB-enriched nutrition had a 59% reduction in CRP (inflammatory marker).
(D) Enhancing Immune Function & Wound Healing
✅ HMB supports immune cell function by maintaining glutathione levels.
✅ Stimulates collagen synthesis → Improves wound healing in post-surgical ICU patients.
📌 Supporting Study:
• Clark et al. (2000): HMB improved wound healing & reduced hospital stay in elderly ICU patients.
2. Clinical Benefits of HMB in ICU Patients
HMB is particularly useful in catabolic ICU conditions where muscle loss is rapid and severe.
✅ Preserves Muscle Mass & Strength
• ICU patients lose 2-4% of muscle mass per day due to immobilization.
• HMB supplementation slows down this muscle loss by inhibiting protein breakdown.
• Deutz et al. (2013): HMB prevented significant muscle atrophy in ICU patients receiving high-protein enteral nutrition.
✅ Reduces ICU-Acquired Weakness (ICU-AW)
• ICU-AW affects 40-50% of critically ill patients, leading to long-term disability.
• HMB supplementation improves functional outcomes (grip strength, mobility).
• NOURISH Trial (2016): ICU patients receiving HMB-enriched oral nutrition supplements (ONS) had a 50% lower 90-day mortality.
✅ Supports Recovery Post-ICU (Rehabilitation Phase)
• Post-ICU patients continue losing muscle for weeks to months after discharge.
• HMB combined with physiotherapy accelerates muscle recovery & reduces re-hospitalization.
• Deutz et al. (2021): ICU patients receiving HMB showed improved physical function at 30 days post-discharge.
3. Landmark Studies Supporting HMB Use in ICU
Study
Key Findings
Clinical Impact
Deutz et al. (2013)
HMB preserved muscle mass in ICU patients receiving high-protein EN.
Supports HMB in ICU nutrition.
NOURISH Trial (2016)
HMB-enriched ONS reduced 90-day mortality by 50%.
Strong evidence for HMB in ICU recovery.
Hsieh et al. (2006)
CRP reduced by 59% in ICU patients receiving HMB.
HMB has anti-inflammatory benefits.
Eley et al. (2008)
HMB increased muscle protein synthesis by 30% in sepsis.
Supports HMB in ICU-acquired weakness.
Clark et al. (2000)
HMB improved wound healing & recovery in ICU.
Useful in post-surgical patients.
📌 Takeaway:
HMB has well-documented benefits for ICU patients, including muscle preservation, inflammation reduction, and improved recovery.
4. HMB Dosage & Administration in ICU
Route
Recommended Dose
Duration
Oral / Enteral (EN)
3 g/day (divided into 2-3 doses)
Minimum 7-14 days, ideally 4-6 weeks
Parenteral (IV HMB not widely available)
Not routinely used
–
📌 Best Administered With:
✔ High-protein EN (whey/casein) → Enhances muscle anabolism.
✔ Omega-3s (EPA/DHA) → Synergistic anti-inflammatory effect.
5. Potential Side Effects of HMB
HMB is very safe, but some mild side effects have been reported:
Side Effect
Frequency
Clinical Relevance
Mild GI Discomfort (bloating, diarrhea)
Rare (<5% of patients)
Dose-dependent, resolves with lower dose
Hypoglycemia Risk (in diabetics)
Low
Monitor blood sugar in insulin-dependent patients
No Liver/Kidney Toxicity
None reported
Safe for long-term ICU use
📌 Safety Summary:
✔ HMB has no major side effects.
✔ Safe in elderly, renal failure, and chronic ICU patients.
✔ Can be continued post-ICU to aid rehabilitation.
6. Final Takeaways
✔ HMB is a potent muscle-preserving agent, working via mTOR activation & protein breakdown inhibition.
✔ HMB reduces inflammation, improving ICU recovery.
✔ HMB supplementation (3 g/day) is recommended in critically ill patients at risk of muscle loss.
✔ Safe, with no major side effects (unlike anabolic steroids).
✔ HMB should be combined with high-protein nutrition + physiotherapy for best results.
Vanhorebeek I, et al. (2020) – ICU-Acquired Weakness Review
🔹 Objective:
• Comprehensive review of ICU-acquired weakness (ICU-AW), its mechanisms, and interventions.
🔹 Key Findings:
• ICU-AW is caused by sepsis, hyperglycemia, neuromuscular blockers, and prolonged immobility.
• 40% of ICU patients develop ICU-AW; in surgical ICUs, this rate is 56-74%.
🔹 Clinical Impact:
• Early mobilization, nutrition (≥1.5 g/kg protein/day), and glucose control are key to preventing ICU-AW.
📌 Reference: Vanhorebeek I, et al. Intensive Care Med. 2020;46(4):637-53
Appleton RT, et al. (2015) – Incidence of ICU-AW
🔹 Objective:
• Systematic review analyzing the prevalence of ICU-AW in critically ill patients.
🔹 Key Findings:
• 4 out of 10 critically ill patients develop ICU-AW.
• ICU-AW significantly delays recovery and increases mortality.
🔹 Clinical Impact:
• ICU patients should undergo early screening for weakness & receive aggressive rehabilitation.
📌 Reference: Appleton RT, et al. J Intensive Care Soc. 2015;16(2):126-36
Wang W, et al. (2020) – ICU-AW & Nutrition
🔹 Objective:
• Reviewed nutritional strategies for ICU-AW patients.
🔹 Key Findings:
• Early enteral nutrition (EN) is superior to PN unless contraindicated.
• Delaying PN for 7 days reduces infection risk (EPaNIC Trial).
🔹 Clinical Impact:
• Reinforces ESPEN guidelines recommending early EN over PN in critically ill patients.
📌 Reference: Wang W, et al. Front Med (Lausanne). 2020;7:559789
Lees et al. (2024) – Muscle Loss in ICU Patients
🔹 Objective:
• Examined muscle wasting trends in critically ill patients across different conditions.
🔹 Key Findings:
• ICU patients lose ~2% of muscle mass per day in the first week.
• 15% muscle loss in 1 week → ICU-AW develops in ~50% of patients.
🔹 Clinical Impact:
• Supports high-protein feeding (1.5-2.0 g/kg/day) & resistance training in ICU.
📌 Reference: Lees et al. Crit Care Clin. 2024
Yuan G, et al. (2021) – ICU-AW & Muscle Mass
🔹 Objective:
• Investigated erector spinae muscle cross-sectional area in ICU-AW patients.
🔹 Key Findings:
• Significant muscle atrophy seen as early as day 7 of ICU stay.
• Worse prognosis in ICU patients with severe muscle loss.
🔹 Clinical Impact:
• ICU teams should monitor muscle loss using imaging & functional assessments.
📌 Reference: Yuan G, et al. Medicine (Baltimore). 2021;100(47):e27806
Prado CM, et al. (2023) – Muscle’s Role in Metabolism
🔹 Objective:
• Analyzed muscle’s role as a metabolic organ & its impact on ICU recovery.
🔹 Key Findings:
• Muscle regulates glycemic control, immune function, and inflammation.
• Severe muscle loss post-ICU increases mortality & disability.
🔹 Clinical Impact:
• ICU recovery strategies must include nutritional & resistance training approaches.
📌 Reference: Prado CM, et al. JPEN J Parenter Enteral Nutr. 2023
He Y, et al. (2019) – ONS & Surgical Site Infection in Orthopedic Patients
🔹 Objective:
• Evaluated whether ONS can reduce postoperative infections and readmission rates in elderly orthopedic patients with hypoalbuminemia.
🔹 Key Findings:
• ONS reduced 30-day readmission risk by 78%.
• Lower incidence of prosthetic joint infection.
🔹 Clinical Impact:
• ONS should be routinely provided postoperatively to improve nutritional status and lower infection risks.
📌 Reference: He Y, et al. J Orthop Surg Res. 2019;14(1):292
Deutz NE, et al. (2021) – ONS & Handgrip Strength
🔹 Objective:
• Studied the effect of ONS on muscle strength (handgrip) in ICU patients with chronic obstructive pulmonary disease (COPD).
🔹 Key Findings:
• ONS improved handgrip strength significantly at day 30 post-discharge.
• CRP (inflammatory marker) levels decreased by 59%, indicating reduced systemic inflammation.
🔹 Clinical Impact:
• Suggests ONS has both anabolic and anti-inflammatory benefits for critically ill patients.
📌 Reference: Deutz NE, et al. Clin Nutr. 2021;40(3):1388-95
Deutz NE, et al. (2016) – NOURISH Trial: ONS & ICU Recovery
🔹 Objective:
• Evaluated the impact of specialized oral nutritional supplements (ONS) on mortality and readmission rates in malnourished hospitalized older adults.
🔹 Key Findings:
• ONS significantly reduced 90-day mortality.
• Improved handgrip strength & body weight at day 30.
• Reduced hospital readmission rates.
🔹 Clinical Impact:
• Supports the use of high-protein ONS in ICU recovery to reduce mortality and improve muscle strength.
📌 Reference: Deutz NE, et al. Clin Nutr. 2016;35(1):18-26